Stanford researchers have used cryogenic 3D imaging at the National Center for X-ray Tomography (NCXT) to identify a new pathway for clearing misfolded proteins from cells. This work presents a potential therapy target for age-related disorders like Alzheimer, Parkinson, and Huntington Diseases.
To deal with these problem proteins, which can disrupt normal functions and can cause degenerative disease, cells sequester them into membraneless inclusions—clusters of misfolded proteins, like tiny garbage dumps—for temporary storage before facilitating their refolding or clearance. But these quality-control mechanisms, and how they change as cells age, are still poorly understood.
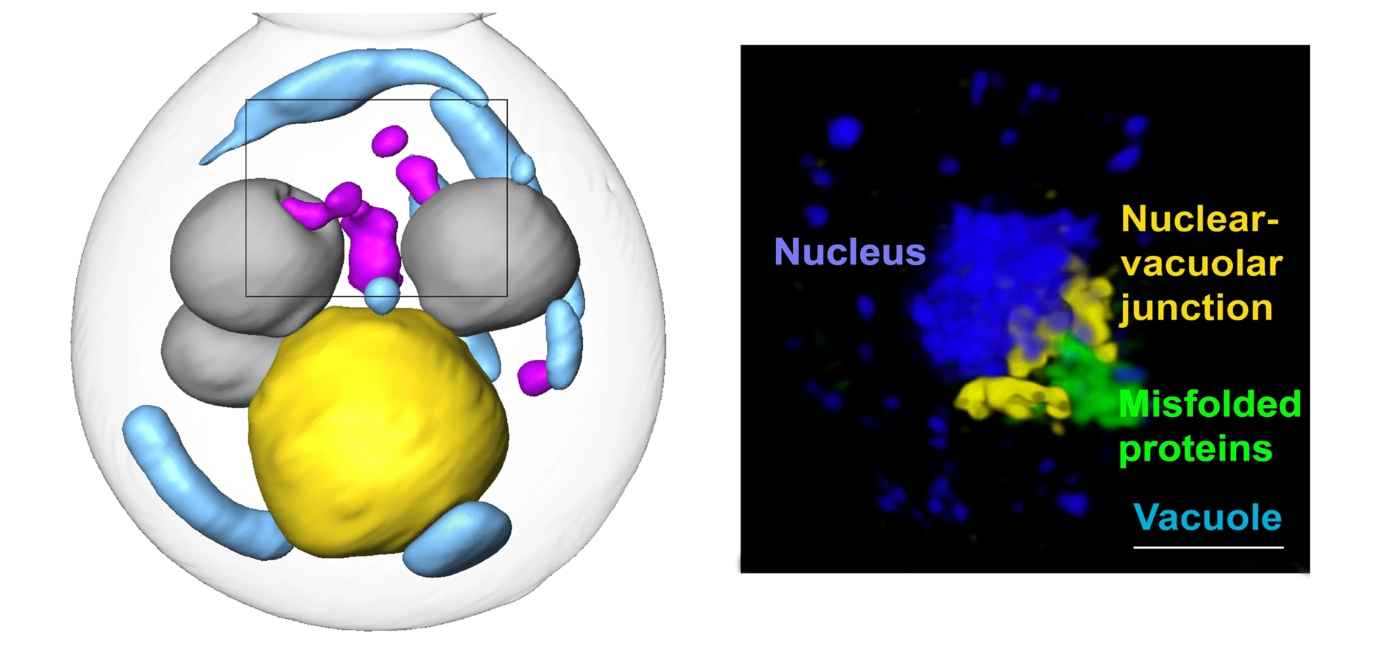
In this new study, published in Nature Cell Biology, researchers illuminated novel spatial and cellular mechanics of this protein quality control process. To do so, the team first restricted misfolded proteins in yeast cells to either the nucleus or the cytoplasm (the area inside the cell but outside the nucleus). The NCXT team included Carolyn Larabell, NCXT Director; Mark Le Gros, faculty scientist in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division; and Jian-Hua Chen, MBIB affiliate scientist. The two groups worked at the Advanced Light Source to combine super-resolution microscopy and soft X-ray tomographic imaging to visually follow the fate of the misfolded proteins.
The researchers found that factors in both the nucleus and the cytoplasm coordinate to move misfolded proteins to a garbage dump site at the intersection of the nucleus and the vacuole—an organelle full of enzymes for degrading proteins—where they are then staged for degradation. While the cytoplasm has a known process for pulling proteins into the vacuole (autophagy), it was unclear how the nuclear inclusions, which were separated from the vacuole by the nuclear envelope, would enter the vacuole. The researchers observed that cytoplasmic inclusions pushed into the vacuole via autophagy, as expected. But the route for the nuclear inclusions was surprising: they budded straight from the nucleus into the vacuole at the junction of the two membranes. Using a series of genetic experiments, the team identified a class of proteins necessary for facilitating that budding-into-the-vacuole action, and thus the entire pathway. These proteins may be attractive therapy targets for misfolded protein diseases.
Going forward, a next step for the researchers is to investigate whether this same pathway is used in mammalian cells to clear human disease-related proteins. Another is to define how communication between the nucleus and cytosol happens along the pathway, and yet another is to see how the pathway is affected by aging.
Read more in the ALS Science Highlight and BioScience News