Fluorescence microscopy is a natural choice when combined with a modality designed for visualizing cell structure. The first-generation Fluorescence Cryo-Microscope (FCM) effectively addressed the challenge of imaging cryopreserved specimens in refractive index-matched fluids. This achievement was made possible by employing cryogenic immersion fluid, such as liquid propane or iso-pentane, instead of air, to enhance the numerical aperture of the microscope. Operating at 10–20 K above liquid nitrogen temperature, the FCM’s design aimed to allow high-quality imaging of cryopreserved specimens while maintaining spatial resolution and light collection efficiency comparable to conventional microscopes. The incorporation of a spinning disk confocal unit further improved performance by reducing contributions from scatter and fluorescence in out-of-focus regions (Cinquin, Bertrand P., et al. “Putting molecules in their place.” Journal of cellular biochemistry 115.2 (2014): 209-216.).
The advantages of FCM over room temperature imaging are manifold. The fluorescence emission spectra of many fluorophores are narrower at cryogenic temperatures, and the working lifetime of fluorescent proteins is significantly extended. This extension, by a factor of 30 or more, enhances signal-to-noise ratio and allows for improved data collection before signal degradation. Moreover, photo-bleaching is less sensitive to the local chemical environment, providing more accurate reflection of fluorophore concentration (Moerner, William E., and Michel Orrit. “Illuminating single molecules in condensed matter.” Science 283.5408 (1999): 1670-1676.). FCM also facilitates tomographic data collection, enabling imaging from multiple angles. The reconstruction of fluorescence tomography data yields isotropic spatial resolution, eliminating the usual 3-fold reduction along the optical (z) axis (Heintzmann, R., and C. Cremer. “Axial tomographic confocal fluorescence microscopy.” Journal of microscopy 206.1 (2002): 7-23.). The overall design strategy of the FCM thus supports high-quality fluorescence imaging at cryogenic temperatures with enhanced resolution and collection efficiency.
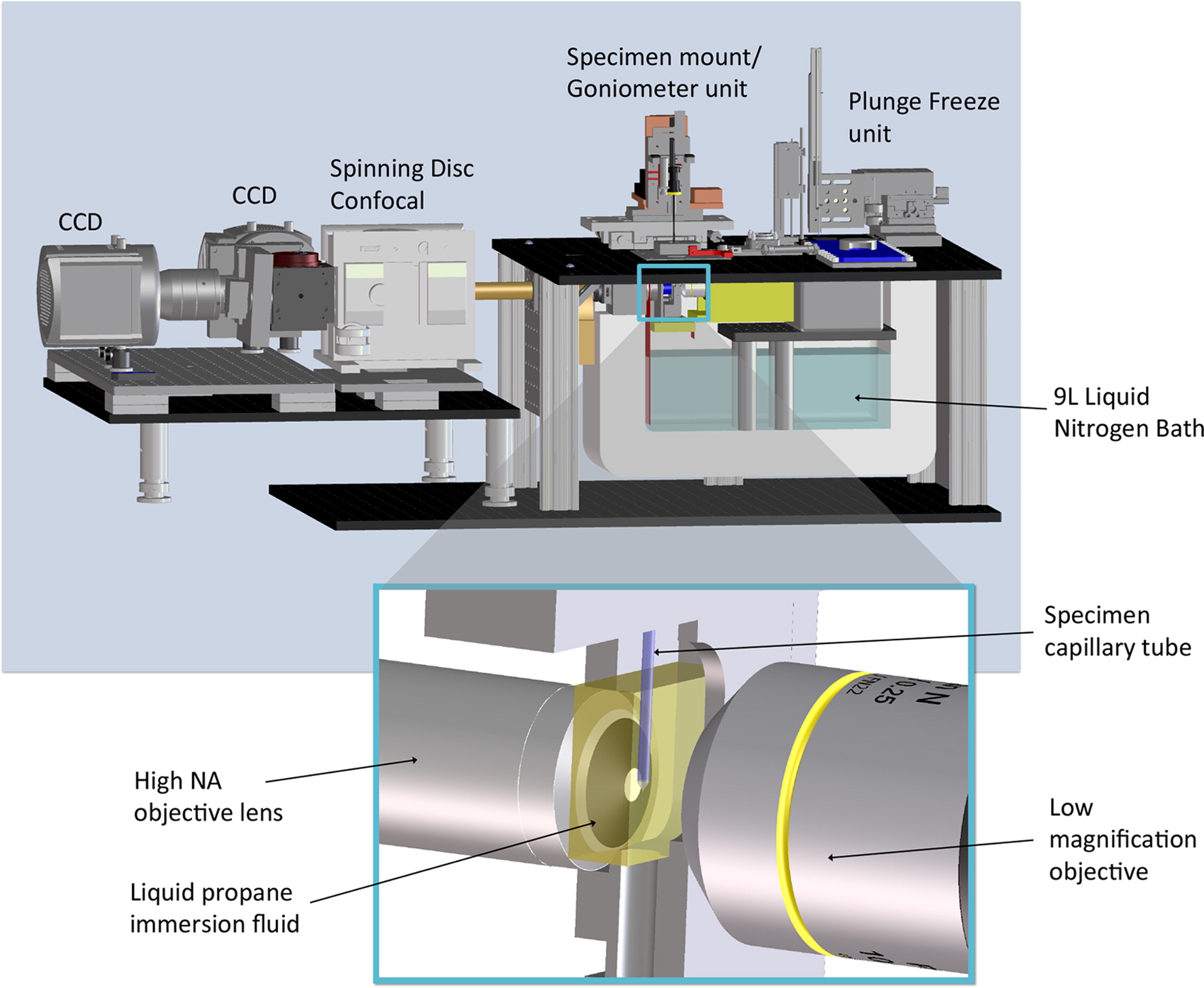
Figure 1:The High Numerical Aperture Fluorescence Cryogenic Microscope (FCM) features a high-magnification side that captures images on Andor EMCCD cameras (red/green channels) through a commercial spinning disk confocal microscope unit. The specimen mount/goniometer, enabling 360° rotation, accommodates thin glass capillaries. A 9 L liquid nitrogen bath maintains stability at ~90 K for several hours without the need for refilling. Rapid cryopreservation is facilitated by swiftly plunging specimens into liquid propane using the device on the microscope’s right side. The inset offers a close-up of the main objective lens with high numerical aperture, a specimen capillary tube in an imaging cell filled with liquid propane immersion fluid, and the objective lens for the low-magnification microscope (Smith, Elizabeth A., et al. “Correlative cryogenic tomography of cells using light and soft x-rays.” Ultramicroscopy 143 (2014): 33-40.).
